Sample questions for exam 2
1) A particle is incident from the left with energy E > V0. Compute the reflection coefficient.
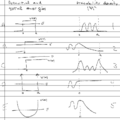
2) Match the figures on the left, with the appropriate one on the right
3) Consider a particle passing over a rectangular potential barrier from left to right. Write down the general form of Ψ(x) in the three regions. Then use the boundary conditions to derive 4 equations for the 5 unknown coefficients. For 10 bonus points, use these 4 equations to derive the transmission coefficient.
4) For a particle in a box, show that the fractional difference in energy between adjacent eigenvalues is
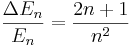
where 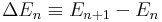
5) Apply the raising operator to the ground state wavefunction for the harmonic oscillator to construct the first excited state wavefunction. Verify by direct calculation that this function satisfies the Schrodinger equation.
6) Let Si,j,i,j = 1,2 be the elements of the scattering matrix. Assume that S is unitary. The matrix equation
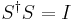 implies 4 scalar equations
implies 4 scalar equations
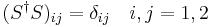
Explain the physical significance of the two equations on the diagonal.
7) Suppose we model the dynamics of a diatomic molecule as a harmonic oscillator. Assume that the frequency of oscillation when in its ground state is 1014 Hz. How much energy (in electron Volts) is necessary to raise the molecule to its first excited state?
8) Show that the stationary states of the harmonic oscillator Hamiltonian are also eigenstates of the operator 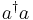 . Explain why this operator is called the number operator.
. Explain why this operator is called the number operator.
9) The wavefunction for a particle of mass M in a one-dimensional potential V(x) is given by
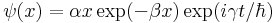 for x > 0 and ψ(x) = 0 for x < 0. Is the particle bound? Explain. What is V(x)?
for x > 0 and ψ(x) = 0 for x < 0. Is the particle bound? Explain. What is V(x)?
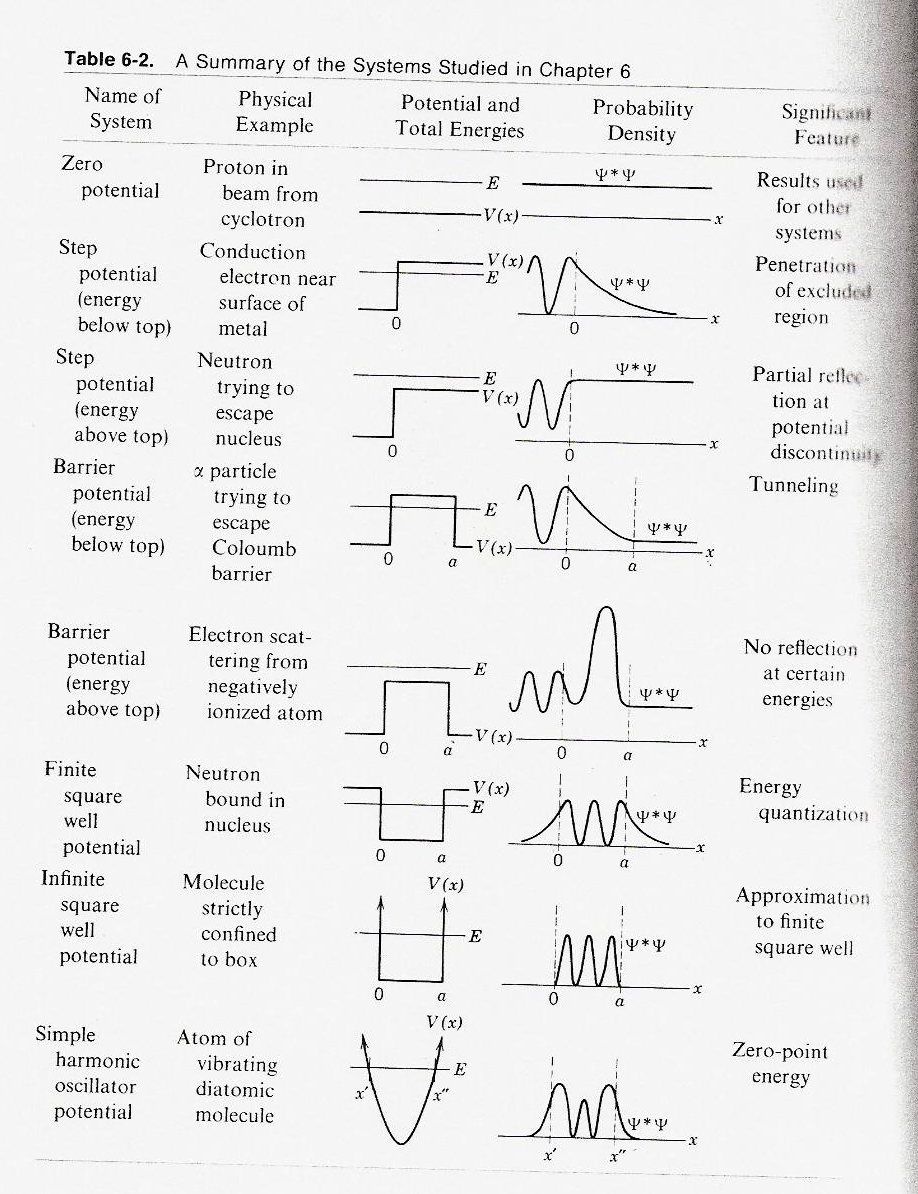
This is table 6-2 from Eisberg and Resnick's text "Quantum Physics ..."