Bnibling/Acoustic Lab
(→Theory) |
m (→Procedure) |
||
| Line 16: | Line 16: | ||
== Procedure == | == Procedure == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 02:46, 4 December 2007
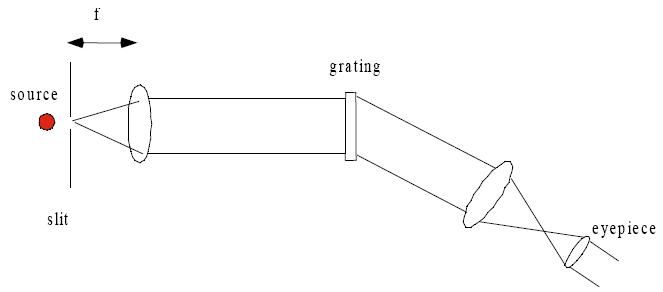
Spectroscopy
Barrett Nibling, Travis Nokes, Kurt Strovink
November 6th, 2007
Contents |
Abstract
List of Figures
Introduction
Theory
Procedure
Results
Helium Results
The following tables and graphs are the results acquired from the Helium Spectroscopy. All published values are from [2].
The First Order Spectrum:
| Color | θdiff (degrees) | λ (nm) | Error (nm) | Published λ (nm) | |
|---|---|---|---|---|---|
| Purple | 15.6 | 448.0 |  2.0 2.0
|
447.148 | |
| Teal | 16.4 | 470.3 |  2.0 2.0
|
471.314 | |
| Green | 17.2 | 492.6 |  2.0 2.0
|
492.193 | |
| Green | 17.5 | 500.9 |  2.0 2.0
|
501.567 | |
| Yellow//Orange | 20.7 | 588.8 |  2.0 2.0
|
587.562 | |
| Red | 23.6 | 666.9 |  2.0 2.0
|
667.815 | |
| Dim Red | 25.1 | 706.7 |  1.9 1.9
|
??? |
The graph above is a comparision between the wavelength calculated using the working equation and the published values, with all values when the error bars.
The following is the first order spectrum of Helium using a 300mm grating
| Color | θdiff (degrees) | λ (nm) | Error (nm) | Published λ (nm) | |
|---|---|---|---|---|---|
| Purple | 7.7 | 446.4 |  4.1 4.1
|
447.148 | |
| Teal | 8.1 | 469.4 |  4.1 4.1
|
471.314 | |
| Green | 8.5 | 492.5 |  4.1 4.1
|
492.193 | |
| Green | 8.6 | 498.2 |  4.1 4.1
|
501.567 | |
| Yellow//Orange | 10.2 | 590.0 |  4.1 4.1
|
587.562 | |
| Red | 11.5 | 664.2 |  4.1 4.1
|
667.815 | |
| Dim Red | 12.2 | 704.0 |  4.1 4.1
|
??? |
Due to the higher amount of error associated with the 300mm grating compared to the 600mm grating, about twice as much, we opted to discontinue any further data collection using the 300mm and stuck with the 600mm for the remainder of the lab.
The Second Order Spectrum:
| Color | θdiff (degrees) | λ (nm) | Error (nm) | Published λ (nm) |
|---|---|---|---|---|
| Purple | 32.5 | 447.5 |  .95 .95
|
447.148 |
| Teal | 34.4 | 470.6 |  .94 .94
|
471.314 |
| Green | 36.4 | 491.6 |  .94 .94
|
492.193 |
| Green | 37.0 | 501.3 |  .93 .93
|
501.567 |
| Yellow//Orange | 44.9 | 588.0 |  .89 .89
|
587.562 |
| Red | 53.3 | 667.9 |  .85 .85
|
667.815 |
In the second order domain, the error is about half of the error in the first order. Even so, all the values when compared to published values are within the error bars.
Sodium Results
The following tables are the result acquired from the Sodium Spectroscopy. Again, the published results are from reference [2].
The First Order Spectrum: In the first order spectrum there are many lines and using the data from the Helium portion of the lab determined that the Sodium has Helium contamination. By substracting all the known Helium lines, all that was left is a bright orange line. By focusing the slit, it was determined to actually be 2 orange lines, this can be seen better in the second order spectrum. Due to the proximity of the lines, less than .1 degrees apart, one measurement was taken and the published values were averaged.
| Color | θdiff (º) | λ (nm) | Error (nm) | Published λ (nm) |
|---|---|---|---|---|
| Orange | 20.7 | 588.8 |  1.99 1.99
|
589.294 |
Second Order Spectrum: In the second order, all the contamination was removed and only the oranges lines were seen.
| Color | θdiff (degrees) | λ (nm) | Error (nm) | Published λ (nm) |
|---|---|---|---|---|
| Orange | 46 | 594.14 |  .89 .89
|
589.294 |
Error Analysis
For the error analysis, there are two variable associated with an error, θd and θi.
The partial errors for each of the variable are calculated from the formulas
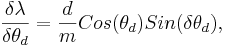
and,
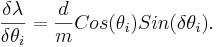
Then the total error is the sum of the two partial derivatives added in quadrature,
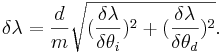
The values for δθd and δθi used are half the value of the smallest unit of measure on the device, .05 degrees.
Conclusion
The emission spectrum for the sodium and helium sources is determined accurately; the error is under 1% and published values fall within that error. The sole exception, the error present in the second order sodium band, is considered to be due to human error during data collection rather than any deviation from the model. The symmetry about θdiff allows each measurement to be confirmed, resulting in confidence in these results.
References
[1]Kowalski et al., Spectroscopy, 2007.[2]Jenkins, F A and White, H E , Fundamentals of Optics, 4E, McGraw-Hill, 1976.